
A couple weeks ago, Sarah and I did a bit of traveling overseas. The day before we left, Sarah called me to let me know she was standing in front of a big bin of crab apples at Berkeley Bowl. I missed crab apples by a few weeks last year, so — wary of the flightiness of bay area seasons — I asked her if she could buy me a few pounds of them. I knew I couldn’t use them immediately, but for this recipe I would be cooking them en sous vide with some salt and sugar before making a puree of them, so I figured freezing them for a few weeks until I was ready to use them would be safe. The crab apple puree would eventually be frozen into a sorbet, then paired with onion jam, white cheddar sauce, olive oil jam, eucalyptus gel, and a black pepper tuile.
On getting back from our travels, I thawed the crab apples for a few days before cooking them in bags in a big stockpot of warm water until they were tender, then pushing them through a tamis to remove the skins and isolate the pulp (an incredibly tedious and hand-cramp-prone process). The mixture gets no more complex than that, though the book directs me to measure the sugar content of the puree with a refractometer before turning it into a sorbet with an ice cream maker. I didn’t previously own a refractometer (and had it in my head that this very exotic-sounding tool would be very expensive), but some searching led me to some pretty reasonably-priced options (they only start getting expensive when you go digital with them) so I snagged one to use here.
The refractometer I bought works by measuring the angle at which light refracts through a given solution that I’m measuring; there’s a small glass plate that I add a few drops of solution on, and a blue plate inside the refractometer that casts a shadow onto a gauge that I look at through a small eyepiece on the device. The line of the blue shadow moves depending on sugar content of the solution; using plain tap water, we can see the line falls at 0 Brix, indicating that the refractometer is calibrated properly (here I’m shooting through the eyepiece with the camera on my phone):
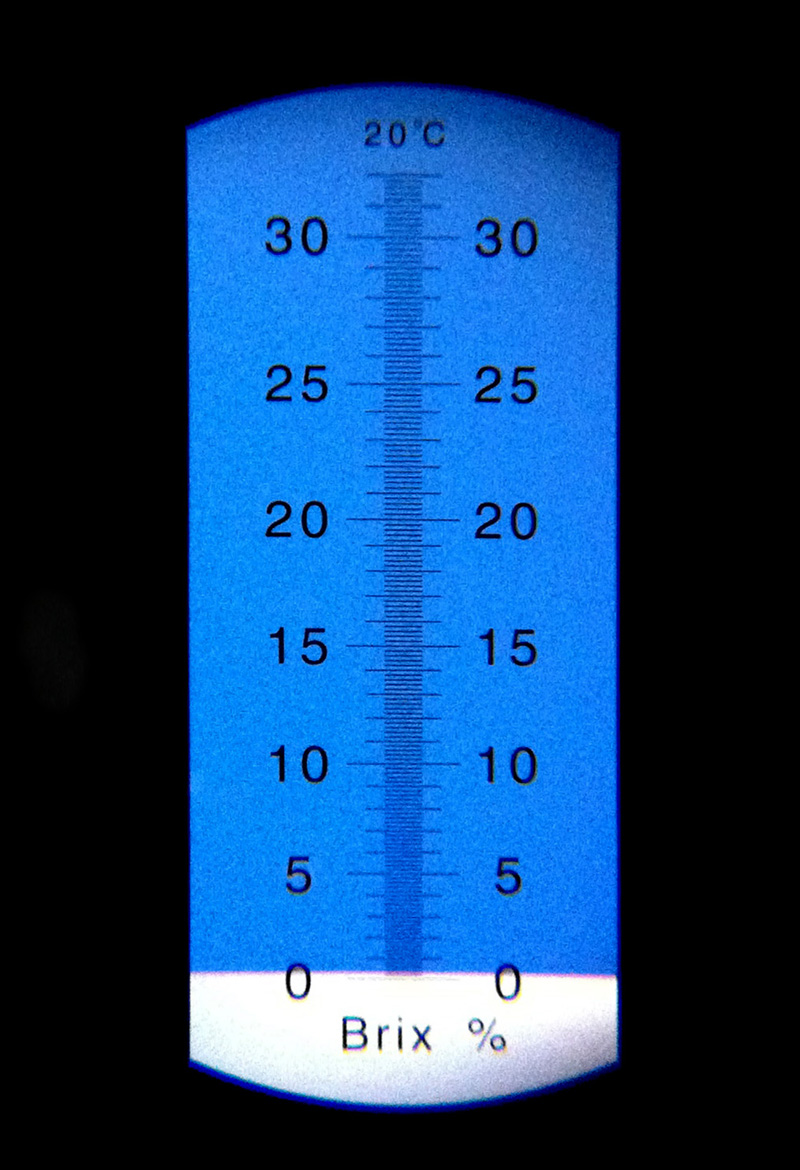
After smooshing my cooked crab apples (and the sweetened juices that resulted from their cooking), I put a few dabs of the puree on the refractometer’s glass and checked again:
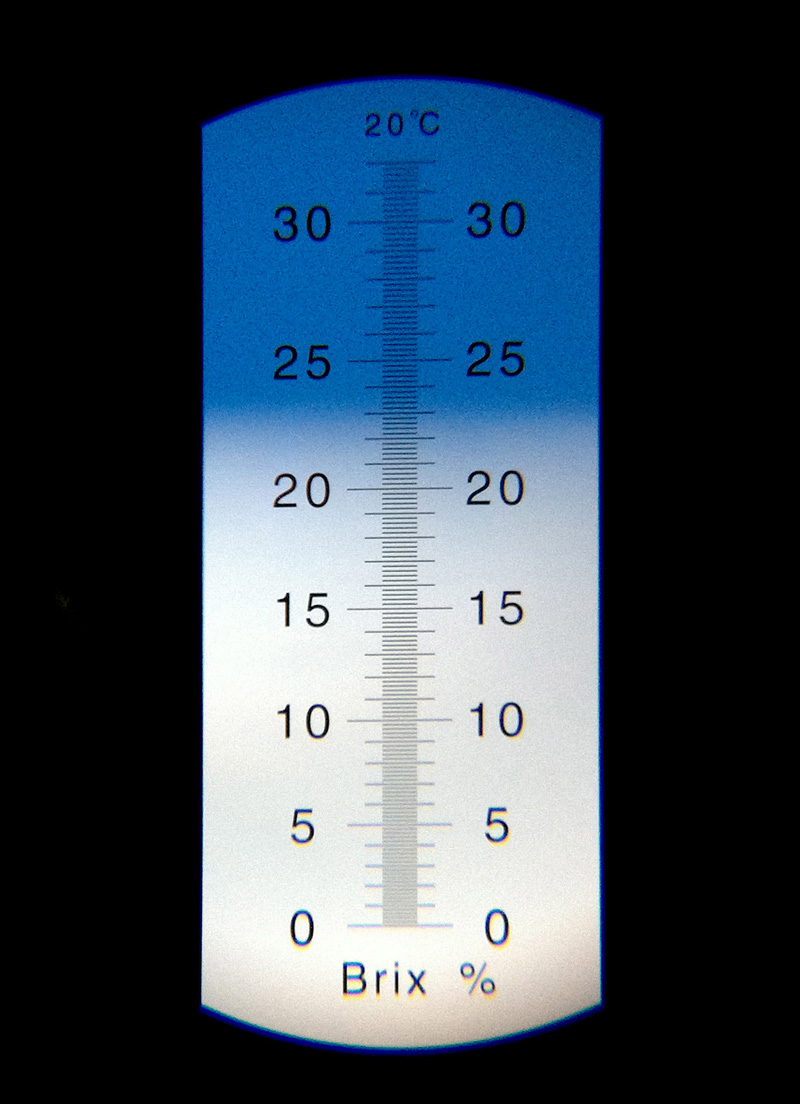
The line is a little fuzzy here because the puree is thick and contains whole cells of the apple’s pulp, but it’s clear that I’m getting a reading above 20 Brix here. I don’t think there’s anything wrong with this (the book says “Add enough sugar so that the mixture reads 20 Brix), it just means my batch of crab apples was particularly sweet already.
My sorbet mixture was, however, a little odd to me; it was more the consistency of smooth applesauce than liquidy, and when I spun it in my ice cream maker it took on an odd, whipped, almost-aerated texture, and it felt grainy/mealy on the tongue when I ate it. It tasted lovely though: tart and sweet and autumnal. But my curiosity was in overdrive, and given the oddness I had with my frozen tamarillos a while back, I wondered if freezing fruits causes overt rupturing of cell walls (and therefore overt introduction of too much pectin into the equation) that was leading to this funky texture.
I went back to Berkeley Bowl and found they still had crab apples, so I bought another couple of pounds, came back home, and remade the sorbet from unfrozen apples. The cooked apples seemed to give off more juice and my resulting puree was notably more liquid, but there was still a bit of an aerated texture and a mealy mouthfeel to things, though again it tasted fine. It’s only occurring to me as I write this that the other common factor with the tamarillo incident is the use of the tamis, and I’m curious if that tool introduces microbubbles into things and/or the grid mesh is coarse enough to lead to a mealy texture. I think I might try thawing the sorbet, blending it at high speed to further break up any cells and liquify it more, and try refreezing it to see if I can get a smoother texture.

Berkeley Bowl’s olive oil aisle is a force to be reckoned with; there are hundreds of different brands and bottles neatly lining the shelves and it’s very easy to get lost. After reading an eye-opening book about olive oil last year, my curiosity is piqued to learn more about the nuances of flavor in them. I have a few brands I know I like, but since I was making an Olive Oil Jam for this dish and knew the delicate flavor of whatever oil I chose would be front-and-center, I took a fair bit of time roaming up and down the aisle trying to pick something nice. I eventually landed on these two, mostly because they seemed to allow for a tightly-contemporaneous tasting. They’re by the same olive oil maker, from the same farm, and were harvested and bottled the same dates.

The two oils are made from two different olive cultivars, and are expelled from pitted olives (a process which claims to remove ‘bitterness’ from the oil). I brought both home and did a side-by-side tasting of them. The first obvious thing I noticed was that they’re both delicious…easily among the better oils I’ve tasted, with a lovely creaminess and a nice bite of pepper at the end that the aforementioned book notes is the sign of a fresh/good oil. But I had to really, really hunt for further differences between the two…which turned out to be eye-opening and kind of fun. I asked for help from Supertaster Sarah, who can usually pick up on subtleties of things that I can’t, and even she had trouble with it. “Tastes like olive oil,” she commented.
There’s a huge part of me that feels that talking about this is incredibly douchey, beyond the pale of foodie masturbation, a real first world problem. But there’s another part of me that wants to think and talk about this meaningfully. So I kept at it, tasting small sips of one, then the other, straining to understand if there were differences and whether any of this mattered. Eventually I got to a point where I could ‘taste through’ the similarities, and could sense the subtle differences between the two. It took a while, and was a bit of a eureka moment for me. The No. 5 was softer, less assertive, less fruity. It was smoother and milder, whereas the No. 3 was a bit brighter, a bit higher in amplitude. But man, you really gotta want it to see that, and for my purposes either of these would work fine. I went with the No. 3, and my olive oil jam turned out very vibrant and delicious.

I went through this same rigamarole to make White Cheddar sauce, a simple sauce made from pouring hot milk over shredded cheese to melt it smoothly. I bought three sharp white cheddar cheeses, one from Black Diamond and two from Cabot, and brought them home to taste them. The differences where were appreciably un-subtle, and I chose the Black Diamond to work with. When I poured hot milk fresh off the boil over the cheese and whisked it to melt, I ended up with a grainy, sandy mixture that didn’t seem to want to smooth out regardless of how much I stirred it. Puzzled by this, I tried again with one of the Cabot cheeses, which yielded the same problem. To the intertubes!
After some searching, I found what I think was the problem; several sites mention grainy melted cheese as the result of too-high melting temperatures. For the third attempt, I let the boiled milk cool for a bit before pouring it over the cheese, then held the bowl over (not on) a burner while I whisked. This turned out much smoother. I can’t really find any technical information about why this happens…is it in Modernist Cuisine? Only time and several more set-aside chunks of my paycheck will tell.

Yet another challenge I ran into was making Eucalyptus Pudding. At first blush, this recipe seems pretty standard-fare for the cookbook: I’m meant to make a sweet gel from water, sugar, citric acid and agar. The sweetness and citric acid offer flavor to what would otherwise be a largely-aromatic thing. The recipe calls for 500g water, 125g sugar, some citric acid and 12g agar. Usually I’ve found agar will gel at a concentration of 0.5%, and most successful recipes in the book use between 0.5% and 1%…any more than this causes trouble. The 12g seemed enormous to me, so I cut it down and went about making everything else as usual. When I let the mixture cool to set, it just didn’t. Surprised, I figured maybe the citric acid had something to do with this. I remelted the mixture, added more agar, and let it set again…and again it failed. I started all over again, this time using even more agar. All told I made this component 5 times, eeking up the agar each time until finally finding that an amazing 42g finally caused the gel to set, albeit in a very odd, pastelike way (rather than a gel, it looked like really thick glue). This was totally fascinating to me; I knew acid had an effect on agar gels but hadn’t seen it in action so fiercly before. As it stood, 42g of agar is enough that you can clearly taste it…at that high of a concentration the gel was not only sweet and tangy but also oddly seaweedy from the agar. For shits I forged ahead with it, adding it the prescribed 10g of eucalyptus oil to flavor it and mixing until it was uniformly incorporated. 10g of eucalyptus oil is about 1/3 of a ‘regular-sized’ bottle of essential oil…in other words, a shit-ton. My kitchen smelled like the Cough Drop Fairy had spontaneously-combusted in it; Sarah, upstairs in our loft, called down, “Whatever you’re doing down there is clearing the hell out of my sinuses”.
Then I tasted it. The mixture was absolutely the most vile, inedible thing ever. The oil is incredibly bitter; this on top of the acidic tang and seaweed flavor made for something that looked like wallpaper glue and just tasted terrible.
Rather than retracing steps that I was pretty sure would lead to the same outcome, I took a step back and decided to go with what I knew. I got a few twigs of fresh eucalyptus, steeped the leaves in just-boiled water for about 20 minutes to make a strong but not bitter eucalyptus tea, then mixed this with sugar, much less citric acid, and a reasonable amount of agar. The omission of an overt amount of acid made the agar behave much better, and using eucalyptus leaves rather than oil got rid of the awful bitterness present in the previous batch. What I ended up with was a clear gel that tasted fresh and minty and sweet, with just the slightest bit of tang to add a little sumpin’ sumpin’ to it.

The other components were thankfully incident-free; I made Onion Jam by slow-cooking a Maui onion in a syrup of sugar and water. I’d read about Maui onions in a Nobu cookbook I have, which mentions their sweetness, and found them at Berkeley Bowl. Having never tried them, I was curious if they’d be recognizably-different from other sweet onions like Vidalias or Walla Walla onions; turns out, not much! But they still yielded a lovely sweet jam that tastes lightly of caramelized onion. And the black pepper tuile was made from a mixture of cooked fondant, isomalt, and glucose with flecks of crushed black pepper sprinkled throughout.
Overall the flavors of most everything were nice, and I found the combinations of the crab apple and onion and olive oil jam to be really delicious. The white cheddar, eucalyptus, and black pepper were a little disparate and odd to me…not un-delicious, but I just couldn’t quite make sense of them.

The Modernist Cuisine’s mac a cheese recipe uses both a emulsifying salt (aka melting salt) and iota keragenan (http://www.consumedgourmet.com/2011/06/modernist-cuisine-at-home-mac-and.html). Modernist Cuisine uses sodium citrate but I’ve seen/heard references sodium phosphates (sodium hexametaphosphate?) in making processed cheese. Check out this paper on “The Melt and Stretch of Cheese” I found online: http://www.cdr.wisc.edu/newsletter/pdf/2000/pipeline_2000_vol12_1.pdf